17/03/2022 – Research results of the Max-Planck-Institute for Iron Research
How hydrogen behaves in aluminium alloys
Due to its low density, high strength, and abundance, aluminium and its alloys are widely used for example in constructions, consumer electronics and for vehicles including cars, ships, trains and planes. However, aluminium alloys are prone to hydrogen embrittlement causing catastrophic failure during service if not noticed early enough. Compared to steel, the effects of hydrogen in aluminium are not well understood.
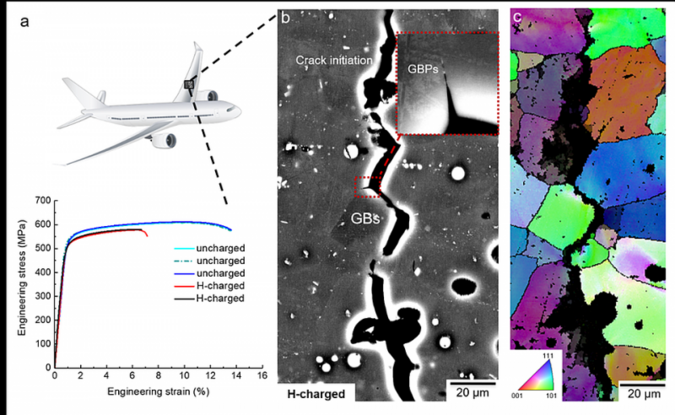
Fig. 1: Tensile properties and metallographic fractography of an aluminium-based alloy with zinc, magnesium and copper after aging for 24 hours at 120°C. a) Engineering stress–strain curves of uncharged and hydrogen-charged samples. b and c) Electron imaging of an intergranular crack of the hydrogen-charged alloy subjected to tensile fracture. © MPIE
Fig. 1: Tensile properties and metallographic fractography of an aluminium-based alloy with zinc, magnesium and copper after aging for 24 hours at 120°C. a) Engineering stress–strain curves of uncharged and hydrogen-charged samples. b and c) Electron imaging of an intergranular crack of the hydrogen-charged alloy subjected to tensile fracture. © MPIE
