18/11/2022 – Flexible, compatible and future-proof
Wire-based stent implants
The demand for stent implants for treatment is increasing in line with the number of cases. At the same time, the areas of application are becoming increasingly diverse and now range from small-lumen applications in the cerebral blood vessels (diameter: < 3mm) to large-lumen areas, for example in the gastrointestinal tract (diameter: > 20mm) [4].
According to the WHO, the leading causes of death worldwide are cardiovascular disease and cancer. In 2016, cardiovascular disease was the leading cause of death worldwide with 17.9 million cases followed by cancer (9 million cases) [1]. From 2007 to 2017, the number of cardiovascular diseases increased by 21.1%. The number of cancer cases increased by 28% from 2006 to 20162, 3. Population growth and increasing life expectancy are cited as the main reasons for the global increase in these diseases. The development of atherosclerosis in blood vessels and tumors near or in vital organs are considered to be the main indications of these conditions.
The dimensions, manufacturing processes and materials vary according to the medical field of application and the cost-effectiveness 5,6. According to the current state of the art, the majority of stent implants are manufactured by laser cutting (approx. 2/3). This is especially true in the small lumen area. In the rapidly growing field of large-lumen applications, implants are predominantly manufactured by wire-based methods (approx. 1/3).
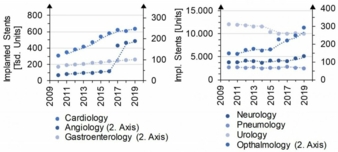
Development of case numbers
In the past decade, the demand for stent implants for the treatment of stenoses, tumors or anastomoses has increased significantly. In addition to the increasing demand in established areas of application such as the vascular system (blood-bearing vessels), the number of procedures in non-vascular areas of application (e.g. gastroenterology, pneumology, urology) is also increasing. There is an increase of 142% and 42% in the vascular and non-vascular fields, respectively. (Fig.1).
Market of wire-based implants
There are currently 79 stent manufacturers and distributors (excluding OEMs) to meet the growing global demand. Most of the relevant manufacturers are based in Germany (21) and the USA (20). In addition, implant manufacturers are located in China (6), Korea (5), India (4), France (3), Israel (3) and Japan (2). The number of products by manufacturer location shows that most products come from the USA (23.3%), Germany (22.6%), Korea (14.9%).
While the use of stent implants in the vascular field for the treatment of stenoses has long been considered the gold standard, they are also increasingly being used in the treatment of tumors or anastomoses. This is also reflected in the number of products available in the various specialties. For example, with 257 products, 58 % of the products available on the market can be found in the field of vascular applications. The other 42% of products are used in non-vascular areas. The manufacturing processes of the implants are as varied as the areas of application. Of the implants currently available, 60.9% are manufactured by laser cutting and 34.8% are wire-based braided (Fig.3).
The braiding process can be further subdivided into the manufacturing approaches single-thread braiding, round braiding with ends closed on one side, conventional round braiding and a mixed form of braiding and winding (cross and hook). Single-thread braiding is the most commonly used manufacturing method of braided products, accounting for 50.3%. This is followed by the cross & hook method with 36.4%, machine circular braiding with ends closed on one side (8.6%), and conventional circular braiding (4.6%).
While cut stent implants are predominantly implanted by balloon dilatation and thus cold-formed to obtain the final, high support force, the braiding process is particularly suitable in large-lumen applications under dynamic load (e.g. carotid) due to the flexible mechanical properties of the product's structures. In the field of vascular applications, 93% of implants are manufactured by laser cutting. Only 5% of the products in this area are braided. In non-vascular applications, 75% of products are braided, 17% are cut, and another 6% are knitted. In the medical specialty area of cardiology, 100% of stent implants are manufactured by laser cutting. In the field of angiology, the figure is only 90%, with 8.7% of stents being braided. In contrast, 80% of implants in the field of gastroenterology are braided, while another 18% are manufactured by laser cutting (Fig.4).
The materials used for the manufacture of stent implants are crucial for the functionality of the products. Accordingly, they are selected and used depending on the addressed specialties. With a share of 61.1%, Nitinol is the most commonly used alloy in current products, which is used in both vascular and non-vascular applications. Furthermore, cobalt-chromium and stainless steel alloys are used especially in the vascular field (Fig. 5).
The dimensions of the stent implants follow the requirements of the indications. Decisive factors in the selection include the diameter and the length of the implant. The evaluation of the product variants by diameter shows that 78% of all product variants fall into the range DStent < 10mm. With 5,527 implants, 43% of all variants are in the smallest diameter range of DStent = 2mm – 4mm. The evaluation of the product variants according to the length of the implants shows 68% of the products in the range of
LStent < 41mm. In terms of diameter and length, a strong standardization of the products can be seen in the range D > 10mm and L > 41mm.
While the data presented here represent the established portion of products and procedures, the market for stent implants continues to evolve with the goal of improved treatment options and optimized, long-term clinical outcomes. Two generations of drug-eluting stents (DES) are followed by degradable implant materials, bioresorbable vascular scaffolds (BVS), and novel manufacturing technologies such as 3D and 4D printing processes [7-9]. While wire-based implants are increasingly gaining acceptance in the global market, the worldwide search for the best solution to treat vascular organ obstructions continues.
The data in this article come from a study conducted at the Institute of Textile Technology at RWTH Aachen University (ITA) on available stent implants on the world market. The focus of the study is on products from market-relevant manufacturers from the USA, the EU and Asia. The result of the research is a data set of 443 products and 12,743 variants (dimension), each with more than 15 product-specific aspects. The full study, entitled "Overview on the Current Global Market of Stent Implants - Market Research 2020 - 21," is available from Shaker Publishing under ISBN 978-3-8440-8536-5.
References
[1] WHO, “World health statistics 2020: Monitoring health for the SDGs, Sustainable development goals,” GPW13, 2020.
[2] Fitzmaurice, C., Abate, D., Abdel-Rahman, O., and Abdelalim, A., “Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study,” JAMA oncology, V. 5, No. 12, 2019, pp. 1749–1768.
[3] Virani, S., Alonso, A., Benjamin, E., and Bittencourt, M. S., “Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association,” Circulation, V. 141, No. 9, 2020, e139-e596.
[4] Stoeckel, D., Bonsignore, C., and Duda, S., “A survey of stent designs,” Minimally invasive therapy & allied technologies : MITAT : official journal of the Society for Minimally Invasive Therapy, V. 11, No. 4, 2002, pp. 137–147.
[5] Bonsignore, C., “SMST 2003: Proceedings of the International Conference on Shape Memory and Superelastic Technologies,” A S M International, Materials Park, 2004, 726 pp.
[6] Stoeckel, D., Pelton, A., and Duerig, T., “Self-expanding nitinol stents: material and design considerations,” European radiology, V. 14, No. 2, 2004, pp. 292–301.
[7] Jiang, W., Zhao, W., Zhou, T., Wang, L., and Qiu, T., “A Review on Manufacturing and Post-Processing Technology of Vascular Stents,” Micromachines, V. 13, No. 1, 2022, p. 140.
[8] Garcia-Garcia, H. M., Serruys, P. W., Campos, C. M., Muramatsu, T., Nakatani, S., Zhang, Y.-J., Onuma, Y., and Stone, G. W., “Assessing bioresorbable coronary devices: methods and parameters,” JACC. Cardiovascular imaging, V. 7, No. 11, 2014, pp. 1130–1148.
[9] Scafa Udriste, A., Niculescu, A.-G., Grumezescu, A. M., and Badila, E., “Cardiovascular Stents: A Review of Past, Current, and Emerging Devices,” Materials (Basel, Switzerland), V. 14, No. 10, 2021.
The authors are Felix Merkord and T. Gries, Institut für Textiltechnik of RWTH Aachen University
Institut für Textiltechnik (ITA)
RWTH Aachen University
Otto-Blumenthal-Strasse 1
52074 Aachen, Germany
Contact person is Felix Merkord
Tel.: +49 241 80-49158
Felix.Merkord@ita.rwth-aachen.de
www.ita.rwth-aachen.de